Blog
Clinical Research
CRA Monitoring Visit Checklist
CRA Monitoring Visit Checklist: Ensuring Clinical Trial Compliance Clinical research associates (CRAs) play a crucial role in the conduct of clinical trials, ensuring that studies are carried out in compliance with regulatory requirements, sponsor protocols, and...
Clinical Trial Emulation
Clinical Trial Emulation: Bridging the Gap Between Observational Studies and Randomized Controlled Trials Clinical research has long been dominated by two primary methodologies: observational studies and randomized controlled trials (RCTs). Observational studies offer...
Decoding Salaries in Clinical Research
Decoding Salaries in Clinical Research In the expansive realm of clinical research, where groundbreaking discoveries are made, and lives are positively impacted, the question of compensation often arises. As the demand for skilled professionals in this field continues...
Software Expertise for Clinical Research Careers
Leveraging Software Expertise for Clinical Research Careers Introduction The intersection of software expertise and clinical research has transformed the landscape of medical innovation. In an era defined by digital transformation, professionals with a strong command...
Job Opportunities in Clinical Research Industry
Job Opportunities in Clinical Research Industry Introduction The clinical research industry stands as a pivotal pillar in healthcare advancement, facilitating the development of new treatments, drugs, and medical procedures. With the rapid expansion of medical...
Exploring Clinical Research Trends in 2024
Exploring Clinical Research Trends in 2024: A Glimpse into the Future of Medicine Clinical research is the backbone of medical advancements, providing the evidence necessary to develop new treatments, improve existing therapies, and enhance patient care. As we step...
Wearables in Clinical Research
Wearables in Clinical Research Introduction In the dynamic landscape of healthcare, technology continues to revolutionize the way we approach clinical research. One of the most impactful innovations in recent years is the integration of wearables into the realm of...
Technology Integration in Clinical Trials in 2023
Technology Integration in Clinical Trials in 2023 : Transforming the Future of Healthcare Introduction In the ever-evolving landscape of healthcare, clinical trials play a pivotal role in advancing medical knowledge and improving patient outcomes. In recent years,...
Digital Health Technologies and Remote Monitoring
Advancing Clinical Research with Digital Health Technologies and Remote Monitoring Introduction Clinical research is the backbone of medical progress, driving innovation in healthcare and ultimately improving patient outcomes. In recent years, digital health...
Precision Medicine in Clinical Research
Precision Medicine in Clinical Research: Revolutionizing Healthcare Introduction In the ever-evolving landscape of healthcare, precision medicine has emerged as a groundbreaking approach that holds the promise of personalized and more effective treatments. Unlike the...
Risk Based Quality Management
Enhancing Clinical Trial Quality through Risk-Based Quality Management Introduction Clinical trials play a pivotal role in the development of new pharmaceuticals and medical treatments. Ensuring their success and reliability is paramount, not only for the health of...
Decentralized Clinical Trials
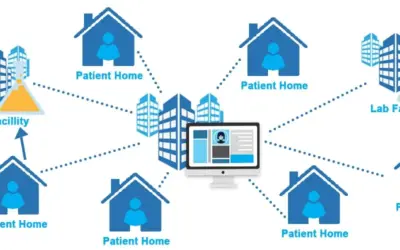
Revolutionizing Clinical Research: The Promise of Decentralized Clinical Trials Introduction Clinical trials are the backbone of medical progress, providing essential data to bring new drugs and medical devices to market. However, the traditional model of conducting...
SOP for Clinical Research Management Plan
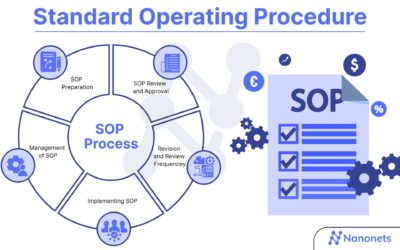
SOP for Clinical Research Management Plan Purpose: The purpose of this Standard Operating Procedure (SOP) is to provide guidelines and instructions for the development and implementation of a clinical research management plan. This plan ensures the efficient and...
Principles of Good Clinical Practices
Principles of Good Clinical Practices Principles of Good Clinical Practices - Role of the FDA In this post we will discuss about the basic principles of Good Clinical Practices and how they apply to clinical trials. Before discussing the Good Clinical Practice...
Oracle Siebel Clinical Overview
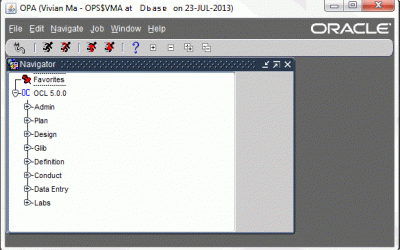
In this post we will be providing an overview of Oracle Siebel Clinical and how organisations use Siebel clinical to manage their clinical trials more efficiently and effectively. Learning Objectives - Oracle Siebel Clinical By the end of this post, you should be able...
Clinical Data Management
Transitioning from CRA to CDM
Navigating the Path: Transitioning from Clinical Research Associate (CRA) to Clinical Data Management (CDM) In the dynamic landscape of clinical research, professionals often find themselves drawn to new avenues for career growth and exploration. For Clinical Research...
Clinical Data Management Trends 2024
Clinical Data Management Trends 2024 Introduction Clinical Data Management (CDM) stands at the forefront of healthcare innovation, ensuring that the wealth of data generated through clinical trials and patient care is harnessed effectively to drive medical...
How is SAS used in Clinical Trials
How is SAS in Clinical Trials: Transforming Data into Lifesaving Insights Introduction The field of clinical trials is a cornerstone of modern healthcare, responsible for bringing innovative drugs and treatments to patients. To ensure the success and safety of these...
Emerging Technology Trends in Clinical Data Management
Emerging Technology Trends in Clinical Data Management Introduction Clinical data management plays a pivotal role in the healthcare industry by ensuring the accuracy, integrity, and security of patient data. As the healthcare landscape continues to evolve, driven by...
SOP for Data Entry in Oracle Clinical
SOP for Data Entry in Oracle Clinical A SOP (Standard Operating Procedure) in clinical trials is a documented set of procedures and instructions that describes how specific tasks or activities are to be carried out in a clinical trial. It provides a standardized,...
Oracle Clinical and RDC upgraded to 5.4.0
Oracle Clinical and RDC upgraded to 5.4.0 We have been offering Oracle Clinical and RDC 5.0.1 software access as part of our Clinical Data Management and Oracle Clinical courses, as well as through our Oracle Clinical - software access subscription service. This has...
Electronic Data Capture
Electronic Data Capture is one of the new technologies have impacted the process of clinical data management and have been well accepted to ensure a more efficient and effective method of data collection. Electronic Data Capture Overview The CDISC defines Electronic...
Processes before Clinical Data Submission
Processes before Clinical Data Submission In this post we shall be discussing about the clinical data management processes and tasks performed before the submission of clinical data to the regulatory authorities. These are basically divided in Data Extract and Data...
Clinical Data Management – Processes after Data collection
Clinical Data Management - Processes after Data collection Clinical Data Management - Processes after Data Collection. After all the data has been collected and the Last Patient Last Visit (LPLV) has been completed, it should be made sure that all the discrepancies...
Clinical Data Management – Processes during Data Collection
Clinical Data Management - Processes during Data Collection Clinical Data Management - Processes during Data Collection. Once the CDMS has been installed and the configured and after the systems have been validated, the process of data collection can begin. The...
Clinical Data Management – Processes Before Data Collection
Clinical Data Management - Processes Before Data Collection Clinical Data Management - Processes Before Data Collection. A good data management method involves the defining of processes for collection of data and its management even before we begin to collect data....
Oracle Clinical Overview
Oracle Clinical Overview Oracle Clinical Overview and its use in Clinical Data Management Oracle Clinical Overview - Oracle Clinical provides the life science industry the most integrated Clinical Data Management (CDM) and Remote Data Capture (RDC) application on the...
Clinical Data Management Systems
Introduction to Clinical Data Management Systems (CDMS) In this article we shall discuss about the overview of clinical data management systems, their origin, definition of a CDMS and its components. This will give you a broad understanding about the use of Clinical...
Clinical Data Collection Methods
Methods of Clinical Data Collection Clinical Data Management requires the collection of raw clinical data. Data collection methods are of three types: Paper-based methods Electronic methods Hybrid methods Paper-Based Methods Paper based methods involve collection of...
Introduction to Clinical Data Management
Introduction to Clinical Data Management The various phases of drug development we talked about in previous blog posts, churn out enormous amount of clinical data which needs to be processed, stored, cleaned and analyzed and finally submitted to the regulatory...
Pharmacovigilance
From Pharmacy to Pharmacovigilance
From Pharmacy to Pharmacovigilance: Navigating the Evolution of Pharmaceutical Safety The world of pharmaceuticals has undergone a dramatic evolution over the past century. This transformation is marked by significant advancements in drug discovery, development, and...
Data Mining Techniques in Pharmacovigilance and the Role of Empirica Signal Software
Data Mining Techniques in Pharmacovigilance and the Role of Empirica Signal Software Introduction Pharmacovigilance, the science of monitoring the effects of medical drugs after they have been licensed for use, is a crucial aspect of ensuring public health safety....
Advent of Automation in Pharmacovigilance
Advent of Automation in Pharmacovigilance In the ever-evolving landscape of healthcare, the advent of automation has been nothing short of transformative. Among its myriad applications, one area that stands out is pharmacovigilance – the science and activities...
Is pharmacovigilance in demand?
Is pharmacovigilance in demand? Exploring the Demand for Pharmacovigilance: A Critical Analysis In the intricate world of pharmaceuticals, where every drug brings hope for better health outcomes, ensuring safety remains paramount. This responsibility falls under the...
10 Reasons to Learn Oracle Empirica Signal
10 Reasons to Learn Oracle Empirica Signal In today's data-driven world, pharmaceutical and life sciences industries are at the forefront of innovation, constantly striving to enhance patient outcomes, optimize operations, and ensure regulatory compliance. Amidst this...
11 Compelling Reasons to Learn Argus Safety
11 Compelling Reasons to Learn Argus Safety Introduction In the realm of pharmacovigilance and drug safety management, Argus Safety stands out as a cornerstone software solution. Developed by Oracle, Argus Safety is a comprehensive platform designed to streamline the...
Navigating Pharmacovigilance Trends in 2024
Navigating Pharmacovigilance Trends in 2024: Enhancing Drug Safety in a Dynamic Landscape Introduction Navigating Pharmacovigilance Trends in 2024. In the realm of healthcare, pharmacovigilance stands as a cornerstone for ensuring the safety and efficacy of...
Pharmacovigilance Market Growth in 2023
Riding the Wave of Pharmacovigilance Market Growth in 2023: Numbers, Trends, and Outlook Introduction Pharmacovigilance, the science and practice of monitoring and assessing the safety and efficacy of pharmaceutical products, is a cornerstone of the global healthcare...
Pharmacovigilance Data management and Eudravigilance
Pharmacovigilance Data management and Eudravigilance The Pillars of Drug Safety: Pharmacovigilance Data Management and EudraVigilance In today's fast-paced world, pharmaceutical companies are constantly striving to develop new drugs and therapies to improve human...
Emerging technologies in Pharmacovigilance
Exploring the Future: Emerging Technologies in Pharmacovigilance Introduction Pharmacovigilance, often referred to as drug safety surveillance, plays a critical role in ensuring that pharmaceutical products on the market are safe and effective for patients....
Signal detection and post authorization safety
Signal Detection and Post Authorization Safety Ensuring Medication Safety Beyond Approval In the realm of pharmaceuticals, ensuring the safety of medications is an ongoing and multifaceted endeavor. While rigorous pre-authorization clinical trials are a crucial step...
Pharmacovigilance Workflows with AI & Automation
Pharmacovigilance Workflows with AI & Automation Enhancing Drug Safety in the Digital Age Pharmacovigilance, the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems, plays...
Big data and AI in pharmacovigilance
Big data and AI in pharmacovigilance Leveraging Big Data and AI for Safer Medicines: Revolutionizing Pharmacovigilance Introduction Pharmacovigilance, the science of monitoring and evaluating the safety of drugs, is undergoing a transformation driven by two powerful...
Patient-centric approaches in Pharmacovigilance
Embracing Patient-Centric Approaches in Pharmacovigilance Introduction Pharmacovigilance, the science and practice of monitoring and assessing the safety of pharmaceutical products, has evolved significantly over the years. Traditionally, this field primarily relied...
Pharmacovigilance in a Pandemic World
Pharmacovigilance in a Pandemic World: Ensuring Safety Amidst Crisis Introduction In a world marked by unprecedented global health crises, pharmacovigilance has emerged as a critical component in safeguarding public health. The COVID-19 pandemic, which swept across...
Medical writing
Understanding Medico Marketing Documents
Understanding Medico Marketing Documents: A Comprehensive Guide Introduction In the fast-paced world of healthcare and pharmaceuticals, effective communication is crucial. Understanding Medico marketing documents play a vital role in bridging the gap between...
Trends in Regulatory Writing
Navigating the Future: Trends in Regulatory Writing Introduction Regulatory writing is a crucial component of the pharmaceutical, healthcare, and biotechnology industries. It involves the creation of documents and reports that communicate complex scientific data and...
The Future of Medical Writing
The Future of Medical Writing: Pioneering a Path to Precision and Accessibility Introduction Medical writing has long been a cornerstone of the healthcare industry, serving as the bridge between complex scientific research and the broader community of healthcare...
Guide to Narrative Writing
This post is a Guide to Narrative writing that will help you understand the importance and various elements of Narrative writing. Introduction The idea of this guide is to introduce the newcomers to the basic writing principles. This is based on the initial writing...
Statistics in Medical Writing
Statistics in Medical Writing In this post, we shall discussion about the significance of Statistics in Medical Writing. We shall look at the components of statistics, the types of data and errors that are commonly encountered while computing them. We shall also learn...
Good versus Poor Scientific Writing
Good versus Poor Scientific Writing In this post, we shall identify what good scientific writing is what is the difference between Good versus Poor Scientific Writing. Its goals and elements will also be explained along with some common myths and misconceptions. Good...
Periodic Safety Update Reports (PSURs)
Periodic Safety Update Reports (PSURs) In this post we shall discuss about Periodic Safety Update reports (PSURs). in this post we will cover the following topics: Origin The origin of PSUR stems from the following: 1992 - "International Reporting of Periodic Drug...
Introduction to Medical Writing
In this post we shall provide an Introduction to Medical Writing, its types and scope in today’s medical world and the in the clinical research industry. Introduction to Medical Writing Medical writing is defined as communicating clinical and scientific data for a...