Planning a Clinical Study in Oracle Clinical is the first step in clinical data management. It helps you define the key parameters of the clinical study as defined with the Protocol. The Planning of a clinical study within Oracle Clinical should start once the draft protocol is ready.
Oracle Clinical is a preferred software used today for the collection and management of raw clinical data generated during clinical trials. It helps handle all the aspects of management of raw clinical data generated during clinical trials.
In this post we shall be discussing about the process of Planning a clinical study using the Oracle Clinical application.
Lets get started!
Table of Contents
The Plan Subsystem
The Plan subsystem of Oracle Clinical is used for planning a clinical study in Oracle Clinical. It is made up of the several modules, where each module performs a subset of activities required for the planning a clinical study.
The modules of the Plan subsystem are as follows:
- Programs
- Organisational Units
- Regions
- Planned Studies
The Plan subsystem along with its respective modules are demonstrated below.
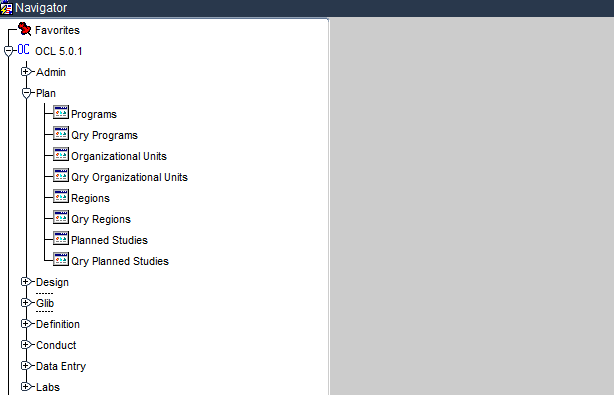
Plan subsystem of Oracle Clinical
The modules help in the movement of data between the tables of the Oracle Clinical database. This means that all data related to planning a study is entered and managed used the above modules. Please note that modules are just triggers, that launch User Interface elements called Forms. It is these Forms that allow the data related to the specific modules to be added, viewed or modified.
Lets talk about each of these modules and their function in planning a study.
Programs Module
The Programs module can be accessed by going into Plan subsystem > Programs
The Programs module helps define the Therapeutic Areas, Diseases and Investigational New Drugs for which you are planning a study. Click on the Programs module and it opens a Maintain Program and Projects form as seen below:
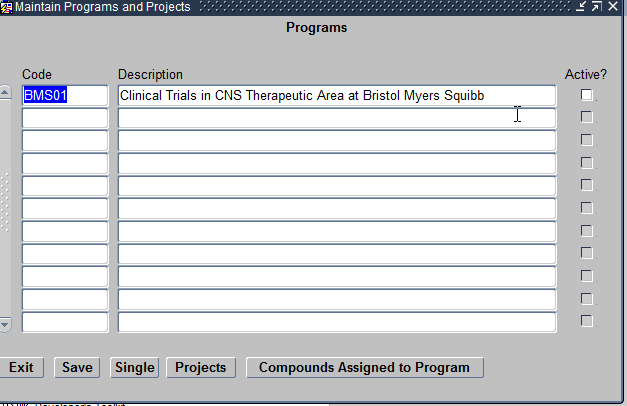
Maintain Programs module
The Maintain Programs and Projects Form allows you to define the following:
- Programs – This refers to the Therapeutic Area being researched by the study. For example Cardiovascular. Each program being created should be unique.
- Projects – This refers to the Diseases under that Therapeutic Area. For example Arrhythmia. You can have any number of Projects under a given Program. Projects within a Program should also be unique.
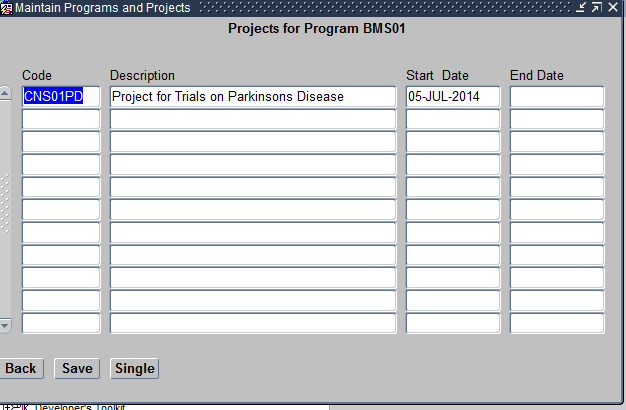
Maintain Projects Form
- Compounds Assigned – These are Investigation New Drugs being researched by the Study being planned. Compounds can be assigned to the Program in this screen.
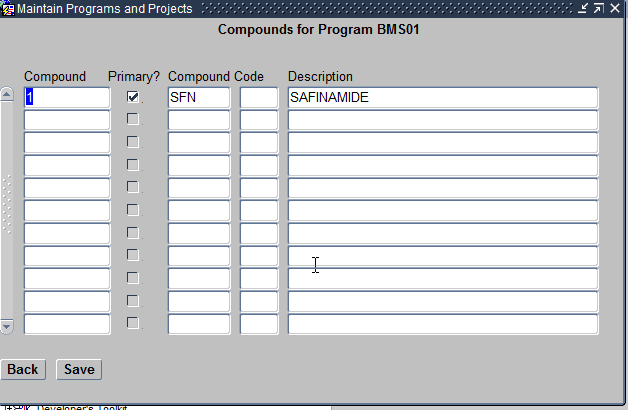
Assign Compounds Form
Please remember that only exisiting Compounds can only be assigned to a Program using this feature.
If you would like to assign a new Investigational Drug to the Program, you would first need to create the same under Design subsystem > Treatment > Active substances as seen below.
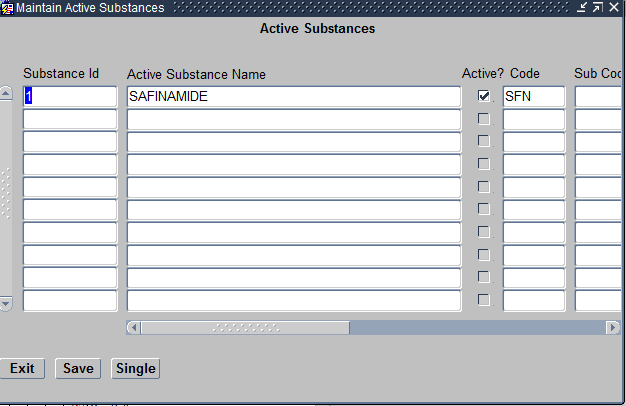
Create Compounds Screen
Organisational Units Module
Organisation units module can be accessed by going into Plan subsystem > Organisational Units
Organisational Units module helps you define the Sponsor of the clinical study. Click on the Organisational units module and it opens a Maintain Organisational Units form as seen below:
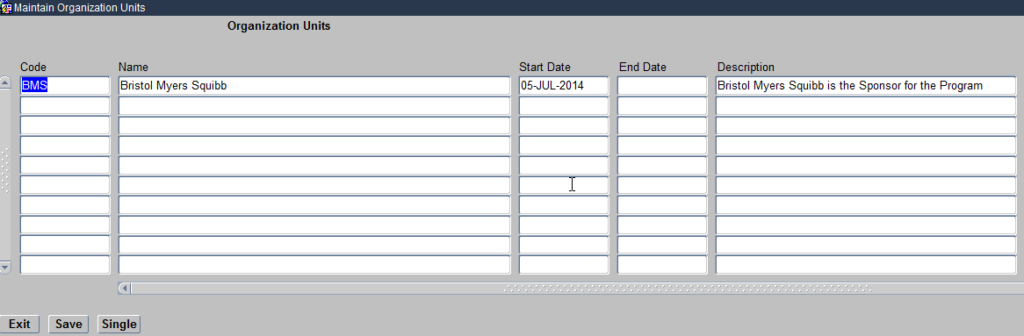
Create Organizational Units
This form allows you to define the Sponsor for the Clinical study being Planned. The Sponsor name should be Unique, which means for example if you see GlaxoSmithKline already listed, because a previous study conducted within Oracle Clinical has been sponsored by this Pharmaceutical company, you should not create it for a new study being sponsored by the same Sponsor.
Hence creating Organisations units is on an as-needed basis and it is not a mandatory step in Study Planning.
Regions Module
The Regions module can be accessed by going into Plan subsystem > Regions
Regions module helps you define the geographical location of Sites where the clinical study will be conducted. Click on the Regions module and it opens the Maintain Regions Form as seen below:
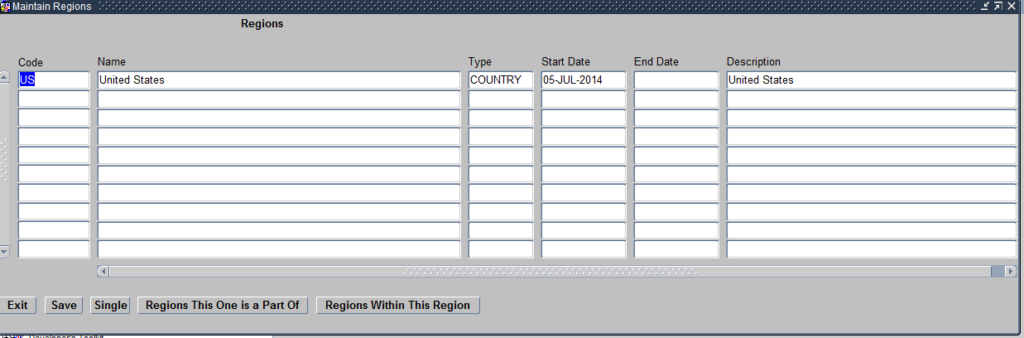
Create Regions
The Form allows you to define the Geographical locations where the Sites will be located. The regions defined in this modules are as per the Site and Investigators Report shared by the Clinical Operations Team and the information in the Protocol.
Please note that here again, the regions should be unique, for example, if United States is already listed, because a previous clinical study was conducted in that region, the the region does not need to be added again, even though it may be valid for the new clinical study being planned.
Regions defined within the Maintain Regions Form can be of the following three types:
- Continent
- Country
- State
The Regions module also has the following Navigation buttons that allow you to create a relationship between the different types of regions as seen below. These buttons are:
- Regions within this Region – For example, select the region United States and click on this button. Then choose Kentucky, that is a state that follows under it.
- Regions this One is Part of – For example, select the region Kentucky and click on this button. The choose United States, that is a Country that it falls within.
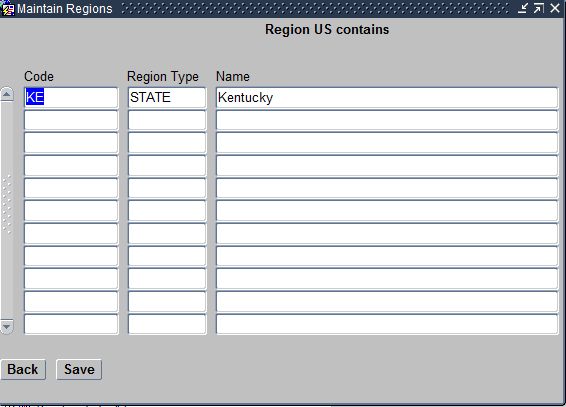
Regions within this Region screen
Thus the Regions module helps create a two way relationship between the Regions defined under the Regions module.
Planned Studies Module
The Planned study module can be accessed by going into Plan subsystem > Planned studies
The Planned studies module brings together the elements you have defined above such a Programs, Projects, Compounds, Organisational units and Regions under the umbrella of a study. Click on the Planned study module and it opens the Maintain Planned Studies Form as seen below:
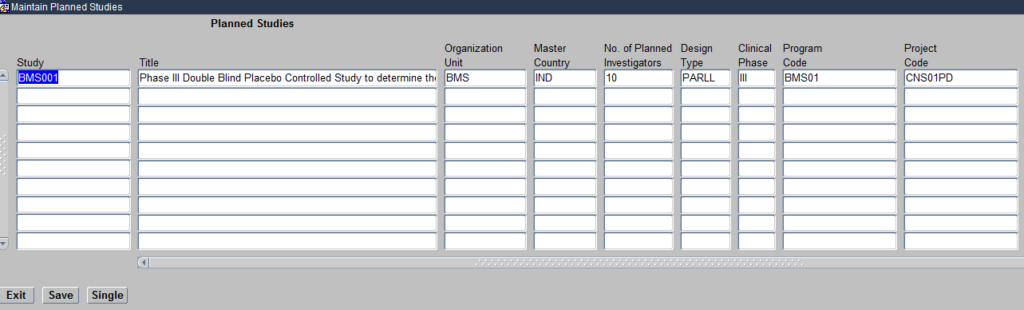
Create Planned Study
The Form allows you to define the various attributes of a Planned Study as seen above
You will notice in the above image, that for each study you define you associate the respective Organisational Unit/Sponsor for that study, the Program/Therapeutic area being researched and the Project/Disease under that Therapeutic Area being researched.
This covers in nutshell the process of Planning a Clinical Study using the Oracle Clinical application.
The Oracle Clinical Program offered by us provides Multimedia tutorials and Recorded lectures on how to Plan a Clinical study.
In our next post we will be covering the process of Designing a Clinical Study within Oracle Clinical
We hope you found this post useful. Please leave your comments below.





An informative one. As a clinical research fresher,I will consider the sugesstion to do the oracle clinical program.