
by ClinSkill | May 14, 2024 | Clinical Data Management
Navigating the Path: Transitioning from Clinical Research Associate (CRA) to Clinical Data Management (CDM) In the dynamic landscape of clinical research, professionals often find themselves drawn to new avenues for career growth and exploration. For Clinical Research...

by ClinSkill | Feb 17, 2024 | Clinical Data Management
Clinical Data Management Trends 2024 Introduction Clinical Data Management (CDM) stands at the forefront of healthcare innovation, ensuring that the wealth of data generated through clinical trials and patient care is harnessed effectively to drive medical...

by ClinSkill | Oct 11, 2023 | Clinical Data Management
How is SAS in Clinical Trials: Transforming Data into Lifesaving Insights Introduction The field of clinical trials is a cornerstone of modern healthcare, responsible for bringing innovative drugs and treatments to patients. To ensure the success and safety of these...

by ClinSkill | Sep 29, 2023 | Clinical Data Management
Emerging Technology Trends in Clinical Data Management Introduction Clinical data management plays a pivotal role in the healthcare industry by ensuring the accuracy, integrity, and security of patient data. As the healthcare landscape continues to evolve, driven by...
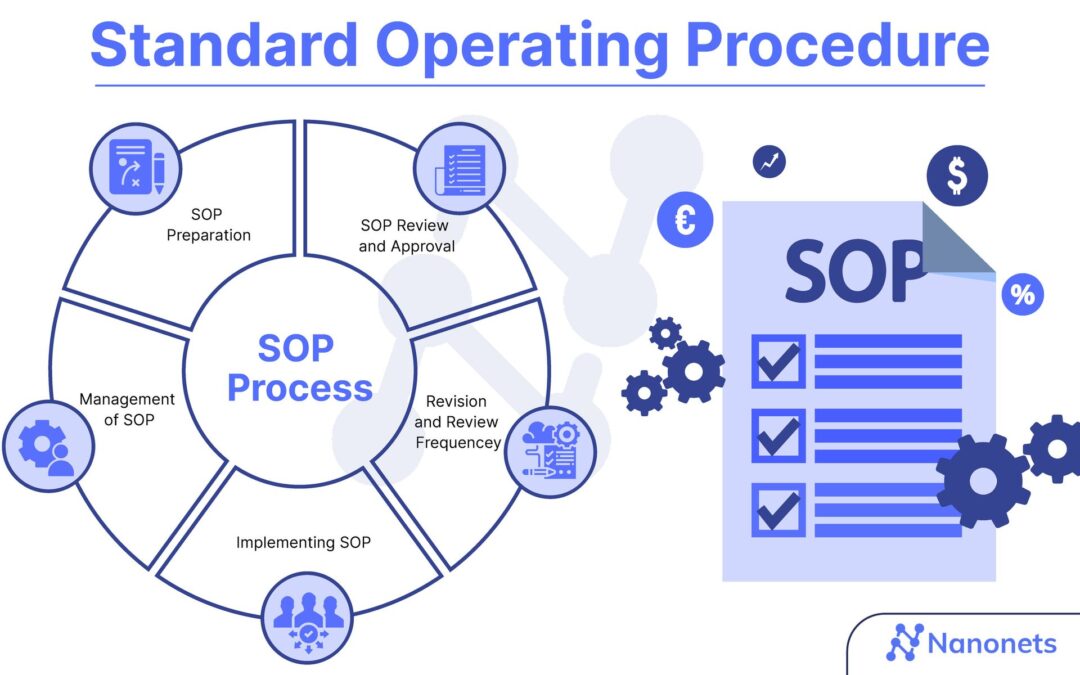
by ClinSkill | Feb 16, 2023 | Clinical Data Management, Oracle Clinical
SOP for Data Entry in Oracle Clinical A SOP (Standard Operating Procedure) in clinical trials is a documented set of procedures and instructions that describes how specific tasks or activities are to be carried out in a clinical trial. It provides a standardized,...

by ClinSkill | Jul 1, 2022 | Clinical Data Management, Oracle Clinical
Oracle Clinical and RDC upgraded to 5.4.0 We have been offering Oracle Clinical and RDC 5.0.1 software access as part of our Clinical Data Management and Oracle Clinical courses, as well as through our Oracle Clinical – software access subscription service. This...







