
by ClinSkill | Oct 5, 2023 | Clinical Research
Advancing Clinical Research with Digital Health Technologies and Remote Monitoring Introduction Clinical research is the backbone of medical progress, driving innovation in healthcare and ultimately improving patient outcomes. In recent years, digital health...

by ClinSkill | Oct 4, 2023 | Clinical Research
Precision Medicine in Clinical Research: Revolutionizing Healthcare Introduction In the ever-evolving landscape of healthcare, precision medicine has emerged as a groundbreaking approach that holds the promise of personalized and more effective treatments. Unlike the...

by ClinSkill | Sep 13, 2023 | Clinical Research
Enhancing Clinical Trial Quality through Risk-Based Quality Management Introduction Clinical trials play a pivotal role in the development of new pharmaceuticals and medical treatments. Ensuring their success and reliability is paramount, not only for the health of...
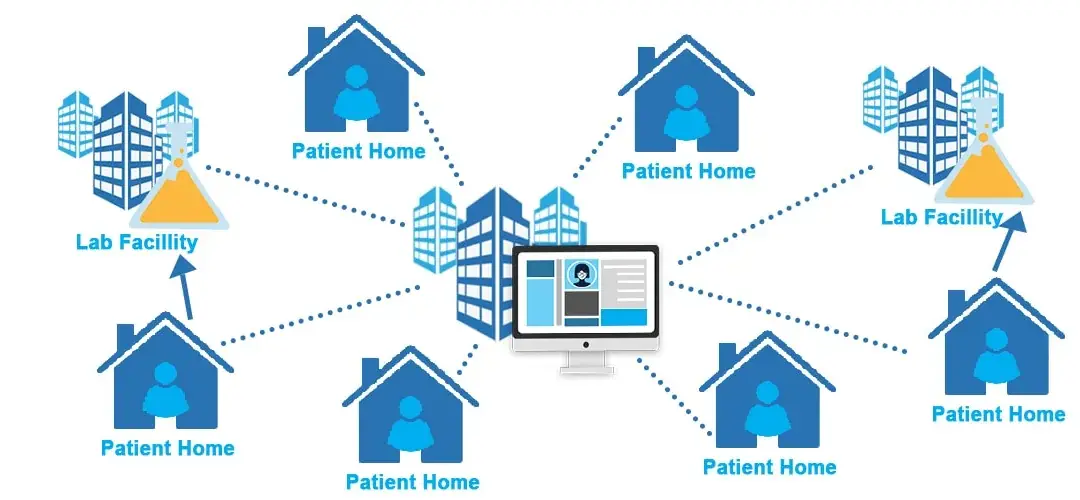
by ClinSkill | Sep 12, 2023 | Clinical Research
Revolutionizing Clinical Research: The Promise of Decentralized Clinical Trials Introduction Clinical trials are the backbone of medical progress, providing essential data to bring new drugs and medical devices to market. However, the traditional model of conducting...

by ClinSkill | Jul 5, 2023 | Clinical Research
SOP for Clinical Research Management Plan Purpose: The purpose of this Standard Operating Procedure (SOP) is to provide guidelines and instructions for the development and implementation of a clinical research management plan. This plan ensures the efficient and...

by ClinSkill | Nov 25, 2022 | Clinical Research
Principles of Good Clinical Practices Principles of Good Clinical Practices – Role of the FDA In this post we will discuss about the basic principles of Good Clinical Practices and how they apply to clinical trials. Before discussing the Good Clinical Practice...






